TITRE is a multicenter trial being conducted in the United States, Canada, and Australia. The goal of TITRE is to determine whether restricting blood transfusion results in less organ impairment and improves long term outcomes for children who receive ECMO support.
About ECMO & Blood Transfusion
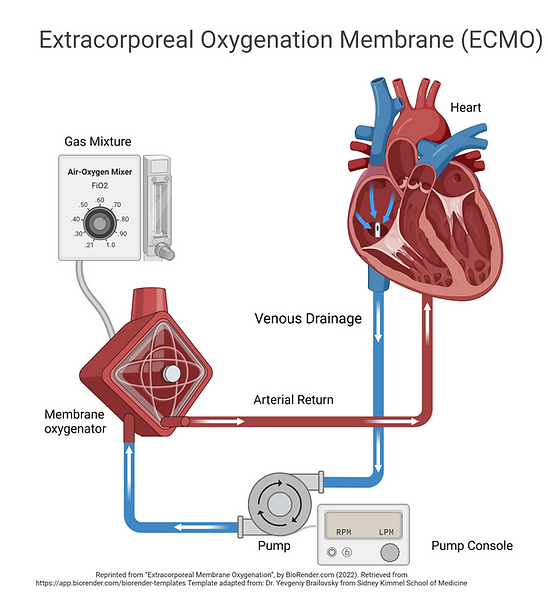
Extracorporeal Membrane Oxygenation (ECMO) is used for heart and lung support. When children are supported on ECMO, they receive a lot of blood transfusions. These are to manage bleeding, and to provide hemoglobin, which is important for carrying oxygen to tissues. Experts believe there may be benefit to using blood transfusions only when there are signs that there is not enough oxygen being delivered to tissues, rather than to target a pre-defined hemoglobin level. In this study, these two different strategies for decision-making around blood transfusion will be studied to see if either strategy is better to prevent organ dysfunction and improve later functional and development outcomes.
Currently, the optimal strategy for blood transfusion in ECMO patients is unknown.
Why TITRE?
Extracorporeal membrane oxygenation (ECMO) can provide life-saving mechanical cardiac and respiratory support to critically ill children, but only about half of children supported with ECMO survive to go home after ECMO. Adverse events are common and long-term function including quality of life may be compromised in many ECMO survivors. TITRE - Trial of Indication-based Transfusion of Red Blood Cells in ECMO, is a multicenter, prospective, randomized clinical trial being conducted to determine whether there is a better way to care for children receiving this invasive support.
What Will We Learn from TITRE?
The goal of TITRE is to determine whether a different decision-making strategy for blood transfusion can reduce organ dysfunction, and improve later neurodevelopment in critically ill children receiving ECMO support.
ELIGIBILITY
Can My Child Participate?
The TITRE Trial completed enrollment in July 2025.
Only centers approved by the funding organization can enroll children in the TITRE trial. If your child is: younger than 6 years old; being considered for ECMO or is on ECMO; and is being cared for by one of the institutions below, please discuss with your provider to see if your child may be eligible for the TITRE trial.
Contact information for each participating institution can be found at clinicaltrials.org. Please contact your local team with questions.
FREQUENTLY ASKED QUESTIONS
What will happen in this research study?
The study lasts 12 months. As part of the study, participants will be randomly assigned to receive blood transfusions according to usual care at the center, or according to the study protocol. We will gather information from participants’ medical records and reach out to participants every 2-3 months after hospital discharge to see how participants’ are doing. Participants will have one additional clinical visit at 12 months after ECMO for neurodevelopmental measurement.
Is there any cost to participate?
Will my privacy be protected?
We take confidentiality very seriously, and will ensure that your right to privacy is protected, and participation in this research study will remain confidential. No names or other identifying information will be used in any published report of information from this study.
Is it possible to leave the study early?
Yes. It is up to the child's family to decide to be in the study. Once in, you may leave the study at any time. Being in the study, or choosing to leave the study, will not affect other medical care at any time.
Tests that are done for research that are not part of regular care will be provided free of charge. Participants and families will be compensated for time and effort.
How long will I be in the study?
Participants are followed for 12 months after enrollment.
Will the results of the study be shared?
We plan to publish what we find out from the study, so we can share this important information with families, researchers, and health care providers. Publications will be announced on this website, and will be communicated through study sites.
Publications
-
Kelly DP, Thiagarajan RR, Alexander P, Bailly D, Baltagi S, Barbaro RP, Bressler E, Friedman M, Guerguerian AM, Kamerkar A, Kim JS, Kozyak BW, Malone M, McGuire J, Perry T, Potera RM, Vitali S, Sleeper LA for the TITRE Trial Investigators. Variation in Blood Product Preparation in Pediatric Extracorporeal Membrane Oxygenation: An Interim Report from the Multicenter TITRE Trial. Transfusion 2024 Sep; 64 (S3):1A-339A.
-
Sleeper LA, Alexander P, Kelly DP, Bembea MM, Bellinger DC, Sadhwani A, Sun L, Shrivastava M, Klein GL, Newburger JW, Thiagarajan RR. Chasing the Dream (of Equipoise): Design and Execution Challenges of the Multicenter TITRE Trial of Indication-Based Red Blood Cell Transfusion in Pediatric ECMO, April 28, 2023. Perfusion 2023; Vol. 38(1S) 82–212, #516
-
Alexander, P.M. Sleeper, LA. O’Halloran, CP, Spinella, PC. Thiagarajan, RR. Change in pSOFA Score as a Novel Outcome for Pediatric ECMO Research, April 27, 2023. Perfusion 2023; Vol. 38(1S) 82–212, #5
-
Sleeper, LA. Alexander, PM. Kelly, DP. Bembea, MM. Bellinger DCB. Sadhwani, A. Sun, L. Shrivastava, M. Klein, GL. Newburger, JW. Thiagarajan, RR. Chasing the Dream (of Equipoise): Design and Execution Challenges of the Multicenter TITRE Trial of Indication-Based Red Blood Cell Transfusion in Pediatric ECMO. J Heart Lung Transplant 2023, 42:4, Supplement, S481.
STUDY TEAM
TITRE Trial Leadership
Clinical Coordinating Center

Ravi Thiagarajan, MBBS, MPH
Trial Co-Chair

Jane Newburger, MD, MPH
Neurodevelopment
Co-Investigator

Peta Alexander, MBBS
Trial Co-Chair

Daniel Kelly, MD, MHQS
Transfusion Medicine/Co-Investigator
Data & Statistical Coordinating Center

Lynn Sleeper, ScD
Grant PI & DCC PI

Anjali Sadhwani, PhD
Neurodevelopment Core/
Co-Investigator

David Bellinger, PhD
Director, Neurodevelopment Core

Melania Bembea, MD, PhD
Medical Monitor
(Johns Hopkins University)
Executive Committee

Heidi Dalton, MD
Inova Children’s Hospital

Marie Steiner, MD, MS
University of Minnesota

Philip Spinella, MD
University of Pittsburgh

Jennifer Muszynski, MD, MPH
Nationwide Children’s Hospital

Christopher Almond, MD, MPH
Lucile Packard Children’s Hospital
Adjudication Committee

Joanne Starr, MD
Children’s Hospital of Orange County

Marianne Nellis, MD, MS
Weill Cornell Medical College

Stacey Valentine, MD, MPH
Univ. of Massachusetts Chan Medical School




















Site Principal Investigators

Erin Bressler, MD
Ann & Robert H. Lurie Children's Hospital of Chicago

Matthew Malone, MD
Arkansas Children's Hospital

Sally Vitali, MD
Boston Children's Hospital

Asavari Kamerkar, DO
Children's Hospital Los Angeles
.jpg)
Sirine Baltagi, MD
Children's Health Dallas

John Kim, MD
Children's Hospital Colorado

Heather Viamonte, MD, MPH
Children's Healthcare of Atlanta

Mina Hafzalah, MD
Children's Hospital of Michigan

Benjamin Koyzak, MD
Children's Hospital of Philadelphia

Andrew Misfeldt, MD
Cincinnati Children's Hospital Medical Center

Kate Ryan, MD
Lucile Packard Children's Hospital

Andrew Smith, MD
Monroe Carell Jr. Children's Hospital at Vanderbilt

Renee Potera, MD
Phoenix Children's Hospital

Matthew Friedman, MD, MS
Riley Hospital for Children
University of Indiana

John McGuire, MD
Seattle Children's Hospital

Marc Anders, MD
Texas Children's Hospital
Baylor College of Medicine

Anne-Marie Guerguerian, MD, PhD
The Hospital for Sick Children

Elise Zivick, MD
MUSC Shawn Jenkins Children's Hospital

Ryan Barbaro, MD, MSc
University of Michigan, Ann Arbor

David Bailly, DO
University of Utah
Primary Children's Hospital

Marino Festa, MD, MBBS
Children’s Hospital at Westmead, Sydney

